Dr. Scharine recently became a certified member of the American Association for Facial Esthetics. The AAFE is an organization that provides advanced training for medical professionals in BOTOX® and dermal filler procedures. Dr. Scharine is now able to give BOTOX® injections for cosmetic purposes and also for therapy. Recently, BOTOX® has been growing in popularity as a treatment for migraines and facial pain. Below are some photos, videos, and other educational material regarding BOTOX® treatments.
- Click here to view The Treatment Visualizer
- Click here to view The Before and After Slide Show
- Click here to view Important Safety Info

What Is BOTOX®?
Clostridium Botulinum
is a rod-shaped bacteria readily found in the soil. There are seven types of
Clostridium Botulinum
bacteria, differentiated by type-naming A through G.
Clostridium Botulinum
toxic types A,B,E, and F cause illness in human begins, although Allergan uses Type A to produce their Botox brand.
Clostridium Botulinum
types B and C cause illness in animals. Incidentally,
Clostridium Botulinum
Type B is used in the production of Myobloc®, another product which has similar effects.
BOTOX® is a therapeutic agent derived from the bacterium, Clostridium Botulinum; Also known as Botulinum Toxin Type A. The brand, BOTOX®, is produced in controlled laboratory conditions and given in extremely small therapeutic doses originally for the treatment of blepharospasm (eye spasm) and strabismus (misalignment of the eye). BOTOX® Purified Neurotoxin Complex is a vacuum-dried form of purified botulinum toxin type A. and is being investigated for several conditions associated with overactive muscle activity.
BOTOX® comes in a vial containing 100u (units) of freeze-dried product. The vials are stored in the office at -5º F until the BOTOX® is usually reconstituted with usually either 2 to 4cc of normal saline. At 2cc reconstitution the dose is 50u per cc or 5u per 0.1cc. At 4cc the dose is 25u per cc or 2.5u per 0.1cc. The results are reportedly best if used within 4 hours of reconstitution. I usually get about 25u in my glabella (in between my eyebrow).
What Is BOTOX
®
Used For?
Other than for its originally intended purpose, treatment of blepharospasm (eye spasm) and strabismus (misalignment of the eye) this product has cosmetic applications as well. BOTOX® is FDA approved for cosmetic use to improve the look of fine lines and superficial wrinkles between the brows caused from every day brow furrowing as well as for migraines. Facial expressions like smiling, frowning or squinting can also cause wrinkles and BOTOX® is very effective in these areas. If you find that the early signs of aging are becoming apparent or if your facial expressions are causing premature wrinkles to appear, then BOTOX® may be for you. BOTOX® is also used in the
prevention
of wrinkles as well, by prohibiting you from making the facial gestures that cause wrinkles.
How Does BOTOX
®
Work?
Normally, your brain sends electrical messages to your muscles so that they can contract and move. The electrical message is transmitted to the muscle by a substance called
acetylcholine
. BOTOX® works to block the release of acetylcholine and, as a result, the muscle does not receive the message to contract. This means that the muscle spasms or movements that cause wrinkling, stop or are greatly reduced after using BOTOX®. Patients sometimes refer to the effects of the injection to their area of treatment as being “paralyzed” temporarily.
Patients with hyperhydrosis (intense sweating) also can temporarily remedy their disorder with BOTOX® injections. The BOTOX® is injected into the areas which sweat profusely like the under arms and backs of knees or palms of the hands, the BOTOX® blocks the release of acetylcholine near the sweat glands which cause the excessive sweating.
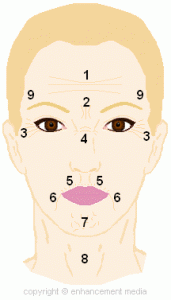
What Areas Can BOTOX ® Be Injected?
1. Forehead Lines: Frontalis Muscle
2. Glabella/Frown Lines: Corrugator Supercilli and Procerus muscles
3. Crow’s Feet (Lateral Orbital Lines): Orbicularis Oculi and Procerus muscles
4. Bunny Lines (Transverse Nasal)
5. Peri-oral Lines (Smoker’s Lines): Orbicularis Oris muscle
6. Marionette Lines: Depressor Anguli Oris and/or Trangularis muscles
7. Mentalis Dysfunction (Chin “Dents”): Mentalis muscle
8. Vertical Platysma Bands: Platysma muscle
9. Brow Lift
BOTOX ® Cosmetic (onabotulinumtoxinA) Important Information
Indication
BOTOX
®
Cosmetic for injection is indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity in adult patients ≤ 65 years of age.
IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING
WARNING: DISTANT SPREAD OF TOXIN EFFECT
Postmarketing reports indicate that the effects of BOTOX
®
Cosmetic and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have an underlying condition that would predispose them to these symptoms. In unapproved uses, including spasticity in children, and in approved indications, cases of spread of effect have been reported at doses comparable to those used to treat cervical dystonia and at lower doses.
CONTRAINDICATIONS
BOTOX
®
Cosmetic is contraindicated in the presence of infection at the proposed injection site(s) and in individuals with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation.
WARNINGS AND PRECAUTIONS
Lack of Interchangeability between Botulinum Toxin Products
The potency Units of BOTOX
®
Cosmetic are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of BOTOX
®
Cosmetic cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method.
Spread of Toxin Effect
Please refer to Boxed Warning for Distant Spread of Toxin Effect.
No definitive, serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX ® Cosmetic at the labeled dose of 20 Units (for glabellar lines) have been reported.
Injections In or Near Vulnerable Anatomic Structures
Care should be taken when injecting in or near vulnerable anatomic structures. Serious adverse events including fatal outcomes have been reported in patients who had received BOTOX
®
injected directly into salivary glands, the oro-lingual-pharyngeal region, esophagus and stomach. Safety and effectiveness have not been established for indications pertaining to these injection sites. Some patients had preexisting dysphagia or significant debility. Pneumothorax associated with injection procedure has been reported following the administration of BOTOX
®
near the thorax. Caution is warranted when injecting in proximity to the lung, particularly the apices.
Hypersensitivity Reactions
Serious and/or immediate hypersensitivity reactions have been reported. These reactions include anaphylaxis, serum sickness, urticaria, soft-tissue edema, and dyspnea. If such reactions occur, further injection of BOTOX
®
Cosmetic should be discontinued and appropriate medical therapy immediately instituted. One fatal case of anaphylaxis has been reported in which lidocaine was used as the diluent and, consequently, the causal agent cannot be reliably determined.
Cardiovascular System
There have been reports following administration of BOTOX
®
of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. Some of these patients had risk factors including pre-existing cardiovascular disease. Use caution when administering to patients with pre-existing cardiovascular disease.
Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junctional disorders (eg, myasthenia gravis or Lambert-Eaton syndrome) should be monitored particularly closely when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including severe dysphagia and respiratory compromise from typical doses of BOTOX
®
Cosmetic.
Human Albumin
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin.
Pre-existing Conditions at the Injection Site
Caution should be used when BOTOX
®
Cosmetic treatment is used in the presence of inflammation at the proposed injection site(s) or when excessive weakness or atrophy is present in the target muscle(s); and when used in patients who have marked facial asymmetry, ptosis, excessive dermatochalasis, deep dermal scarring, thick sebaceous skin or the inability to substantially lessen glabellar lines by physically spreading them apart.
ADVERSE REACTIONS
The most frequently reported adverse events following injection of BOTOX
®
Cosmetic include blepharoptosis and nausea.
DRUG INTERACTIONS
Co-administration of BOTOX
®
Cosmetic and aminoglycosides or other agents interfering with neuromuscular transmission (eg, curare-like nondepolarizing blockers, lincosamides, polymyxins, quinidine, magnesium sulfate, anticholinesterases, succinylcholine chloride) should only be performed with caution as the effect of the toxin may be potentiated. Use of anticholinergic drugs after administration of BOTOX
®
Cosmetic may potentiate systemic anticholinergic effects.
The effect of administering different botulinum neurotoxin serotypes at the same time or within several months of each other is unknown. Excessive neuromuscular weakness may be exacerbated by administration of another botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin.
Excessive weakness may also be exaggerated by administration of a muscle relaxant before or after administration of BOTOX ® Cosmetic.
USE IN SPECIFIC POPULATIONS
BOTOX
®
Cosmetic is not recommended for use in children or pregnant women.
Please see BOTOX ® Cosmetic full Prescribing Information including Boxed Warning and Medication Guide .




Follow Us Online